COMPLIANT. EFFECTIVE. EFFICIENT.
In a globally regulated life science industry, PathWise provides proven methodologies in quality and compliance through hands-on and practical solutions that ensure compliant, effective, and efficient quality systems. Through virtual instructor led and on-demand online modules, we help simplify necessary concepts and processes, ensuring a quality system program that conforms to industry and regulatory standards.
INSTRUCTOR LED VIRTUAL TRAINING
PathWise instructor led training allows feedback & dialogue between the attendee and instructor, while working through problems in the safety of the classroom environment. Our virtual training environment has the same interactivity and engagement as the classroom, with the safety of learners participating from their homes.
ONLINE TRAINING THROUGH ePATH
PathWise online learning allows for training flexibility, teaching rules, definitions, and regulation requirements. The self led modules include interactive learning checks, and helpful downloads and tools to support your quality system needs.
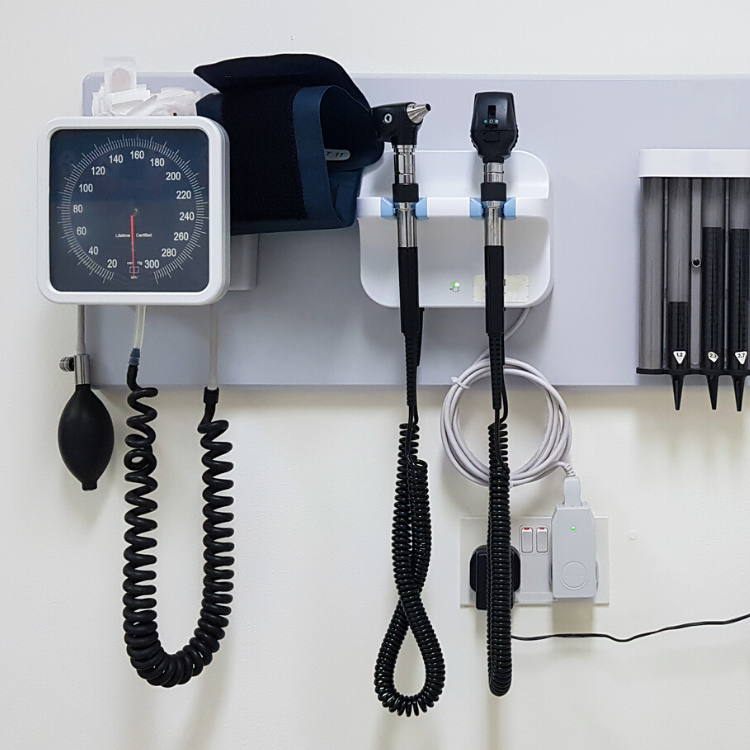
Medical Device
Full understanding and compliance with the Quality System Regulation (QSR) is key for your organizations success. Whether it is CAPA training, supplier auditing, or any other quality system need, PathWise has the experience to help.
Pharma
Developing a systematic, compliant approach to current Good Manufacturing Practices (cGMP) and 21 CFR parts 210 & 211 isn’t easy. PathWise provides tools, systems, and best practices to simplify regulatory compliance for Pharmaceutical companies.
Biologic
Biologic and biotechnology regulations are becoming increasingly rigid, and having the right support to understand and comply with 21 CFR parts 600, 601, and 610 can make all the difference.
FROM OUR LEARNERS
QUALITY SYSTEMS and compliance training solutions.
For over 20 years PathWise has delivered superior training, to simplify necessary concepts and processes in your quality systems.
ePath Users
Instructor Led Learners Trained Annually
Online Training Course titles
Virtual Instructor Led Course Titles
Countries Trained For

EARN continuing education credit
PathWise is accredited by the International Association for Continuing Education and Training (IACET). PathWise complies with the ANSI/IACET Standard, which is recognized internationally as a standard of excellence in instructional practices. As a result of this accreditation, PathWise is accredited to issue the IACET CEU.
COMPANIES WHo choose pathwise
PathWise has trained and supported some of the top organizations in the Life Science industry.


















